

Parents around the globe are using pediatric OTC medications to relieve their children’s pain, cold and allergy symptoms, and to reduce fevers. When parents give these medications to their infants and children, they expect the products to be free from harmful industrial chemicals.
At Clean Label Project® , rather than accepting safety as a given, our analysis is based on data and scientific evidence to uncover the actual substances consumers are ingesting, focusing on promoting and safeguarding transparency.
This study serves as a wake-up call for consumers, manufacturers, retailers, and regulators alike. With the lack of comprehensive federal regulations specifically addressing phthalates in pediatric OTC medicines, it is critical that the industry independently takes proactive measures to address the issue. Clean Label Project®’s findings call for a new level of transparency and stricter safety standards to protect consumers from long-term exposure to these contaminants.
This report seeks to initiate a critical discussion on the safety of pediatric OTC products, empowering consumers to make better-informed decisions and encouraging manufacturers to prioritize the purity of ingredients and packaging. By exposing these hidden risks, Clean Label Project® advocates for an industry-wide commitment to cleaner products.
Of the 40 products (from 32 of the Top Brands) that CLP Tested:
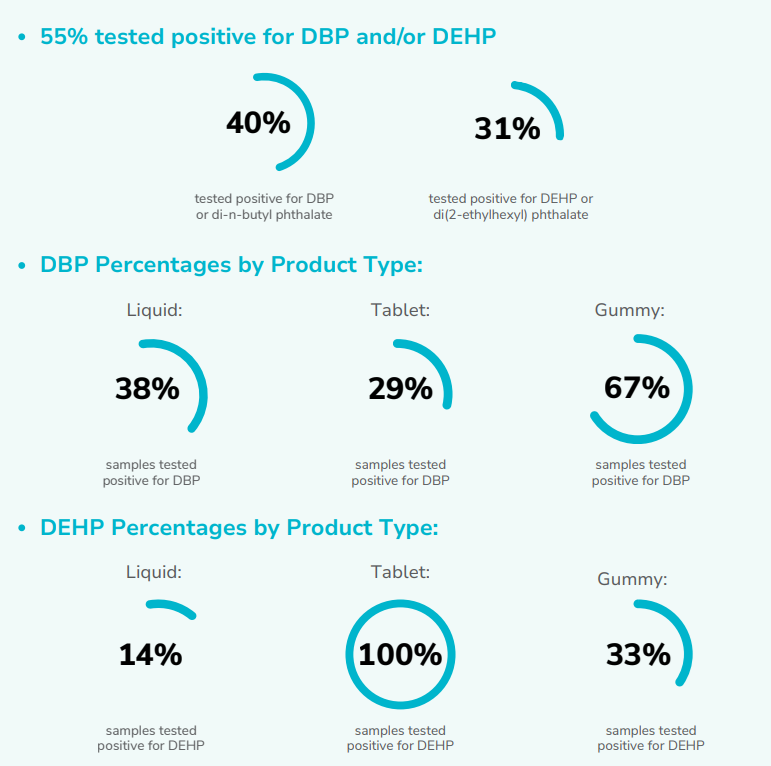
DBP and/or DEHP Percentages by Product Type:
Clean Label Project® purchased and rigorously tested 40 of the top-selling pediatric OTCs, sourced from Nielsen and Amazon’s best-seller lists, and supplemented with top-selling products from the natural and organic marketplace.
The scope of testing assessed multiple panels of industrial and environmental contaminants. Collaborating with Ellipse Analytics, an ISO 17025-accredited analytical chemistry lab, Clean Label Project® amassed 4,880 data points from those 40 products, representing 32 brands, to benchmark the findings.
Pediatric OTCs evaluated by Clean Label Project® showed detectable levels of DBP (di-n-butyl phthalate), and DEHP (di(2-ethylhexyl) phthalate).
In December 2011, the National Institutes of Health (NIH) published a study of DBP and DEHP in Prescription and Over-The-Counter Medications, which led the FDA to create new guidelines in December 2012, for the pharmaceutical industry to limit usage of DBP & DEHP phthalates.
Discovering DBP and DEHP in more than half of our pediatric OTC samples prompted us to investigate the various pathways through which these phthalates enter OTC products.
There are no comprehensive federal regulations specifically addressing dietary exposure to industrial chemicals in food and medicine, as most safety measures concentrate on physical and microbiological contaminants.
While there are FDA guidelines for DBP and DEHP to limit the usage of these phthalates in pharmaceuticals, there are no actual federal restrictions on the usage. Clean Label Project® performs 8-12 category studies annually, and we find a high percentage of samples contain phthalates. DEHP appears on the FDA’s GRAS list and, due to its role as a plasticizer enhancing flexibility, is commonly found in products like bottles, bags, and gaskets.
Over the past decade, the Clean Label Project® has analyzed bisphenols (BPA & BPS) across a range of product categories. While BPA was frequently found in earlier studies, it is now rarely detected. We expect phthalates like DBP and DEHP to experience a similar decline over time.
DBP (di-n-butyl phthalate) and DEHP (di(2-ethylhexyl) phthalate) can contaminate pediatric OTC products through several different pathways.
DBP is often used as a coating for oral medications, particularly those for timed-release or delivery to the large bowel.
DEHP is a plasticizer that makes plastics more flexible. DEHP plastic chemicals are used in bottles, bags, and plastic rubber seals. DEHP is part of the FDA’s GRAS (Generally Recognized As Safe) list. Our research has found DEHP in many different categories we’ve tested, such as protein powder, cereal, and snack bars.


Clean Label Project® contracted with an independent analytical chemistry laboratory, Ellipse Analytics, to test five industrial chemical panels:
The Heavy Metals (Arsenic, Cadmium, Lead, and Mercury) were tested by Inductively Coupled Plasma – Mass Spectroscopy (ICP-MS).
Phthalates were tested by Gas Chromatography – Mass Spectroscopy (GC-MS/MS).
Bisphenols, Glyphosate, and Pesticides are tested by Liquid Chromatography – Tandem Mass Spectroscopy (LC-MS/MS).
Our mission is to empower consumers by delivering accurate and reliable product information based on our testing and analysis of the products they buy. When it comes to selecting low-contaminant options, our findings reveal that liquid pediatric OTCs have lower phthalate contamination than tablets or gummies.
Clean Label Project® believes DBP and DEHP are entering pediatric OTCs as an ingredient to slow the release of medication and as plasticizers in the manufacturing of capsules and packaging.
Over the last couple of years, 90% of the samples tested in Clean Label Project® category studies were BPA free. In each study, we may find one or two samples that are outliers, where we find a spike in BPA. Clean Label Project® finds phthalates like DBP and DEHP much more frequently.
This data enables consumers to make informed choices that prioritize safety and quality in their Pediatric OTC choices.
“When it comes to our children, standards should always be set as high as possible,” said Molly Hamilton, Executive Director of Clean Label Project®. “Parents provide medication specifically to safeguard their child’s health, and we must do everything possible to ensure it accomplishes that safely and effectively.”
The following companies have Pediatric OTCs that are Clean Label Project® Certified:

Clean Label Project purchased and rigorously tested 160 of the top-selling protein powders, sourced from Nielsen and Amazon’s best-seller lists, and supplemented with top products from the natural and organic marketplace. It also assessed multiple panels of industrial and environmental contaminants. Collaborating with an analytical chemistry lab, Clean Label Project amassed 35,862 data points from 70 brands and 160 products to benchmark the findings.
Protein powders tested by Clean Label Project had an array of positive results for levels of arsenic, cadmium, lead, and mercury. However, 47% of products exceeded at least one federal or state regulatory set for safety, including CA Prop 65, and 21% of the samples were over 2X CA Prop 65 levels.
– Heavy metals, such as arsenic, lead, mercury, and cadmium, are naturally occurring elements found in the Earth’s crust.
They enter the environment through natural processes like volcanic eruptions, weathering of rocks, and soil erosion. Over time, they accumulate in air, water, and soil, where they can make their way into plants, animals, and eventually into human food sources. Though naturally occurring, the concentration of these metals can increase due to human activities such as mining, industrial processes, and agricultural practices, leading to higher exposure risks in food products. Given the absence of federal regulations that require proactive testing to minimize the introduction into finished products, they can be unintentionally introduced into all foods and consumer products. Interestingly, certified organic products were found to have, on average, three times the lead compared to non-organic products. This was largely due to plant-based protein powders, which tend to contain higher levels of contaminants. Our studies continue to report chocolate as a high-risk ingredient.
– 65% of Chocolate Protein Powders tested over Prop 65 levels and 29% tested over 2X Prop 65.
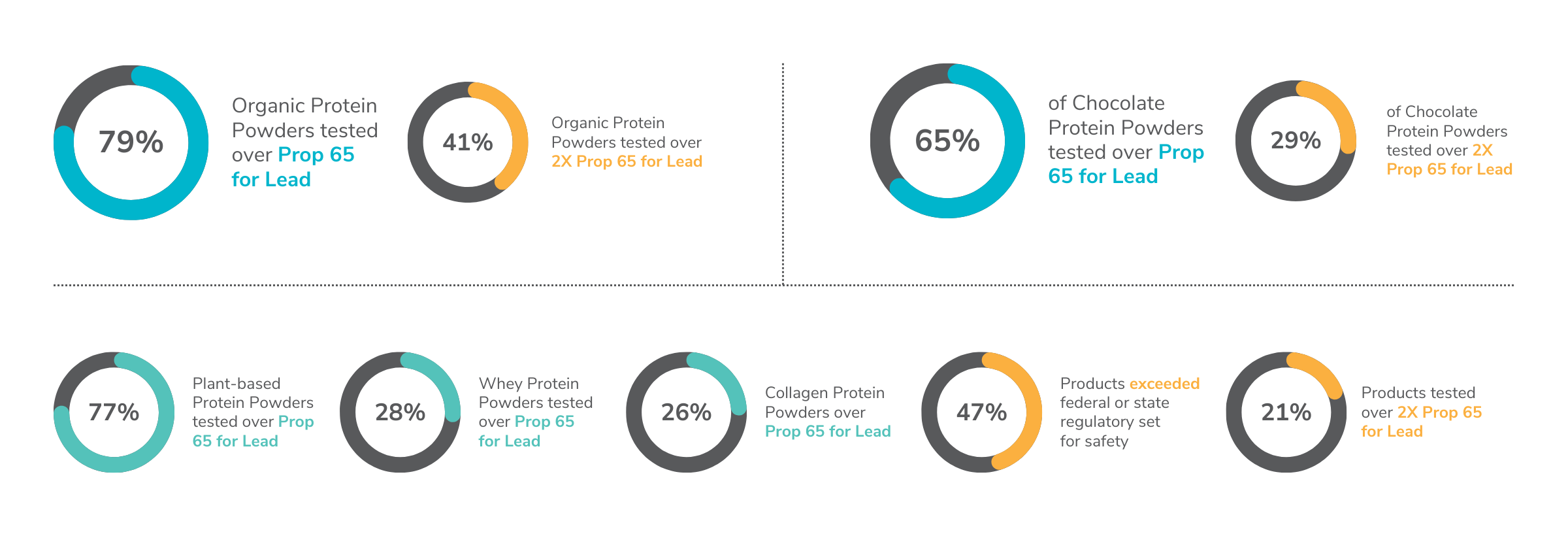
Bisphenols, including BPA and BPS, are well-known endocrine disruptors with significant health risks. Research has shown that BPA can interfere with insulin tolerance, potentially undermining athletic training efforts for those using protein powders (Moon et al., 2015). Additionally, BPA is linked to metabolic issues, increasing the risk of type II diabetes (Le Magueresse-Battistoni et al., 2018). Fortunately, Clean Label Project’s 2023-2024 study found a significant improvement from 2018, with BPA and BPS detected in only 3 of 160 protein powder products, compared to 55% in previous tests. Plant-based protein powders were the most contaminated, containing five times more cadmium than their whey-based counterparts. Even the flavor of protein powder played a significant role in contamination levels. Chocolate protein powders, for instance, were found to have a staggering 110 times more cadmium [1] than vanilla-flavored varieties. Meanwhile, whey-based protein powders generally showed much lower contaminant levels, highlighting the variability in product safety depending on the protein source and flavoring.
Surprisingly, there are no comprehensive federal regulations specifically targeting dietary exposure to heavy metals in food, with most safety efforts focused on physical and microbiological contaminants. However, recent discussions in Congress and the FDA are pushing for stricter standards on heavy metals and industrial chemicals in food products. States like California have created regulations, like Prop 65 that we reference. Prop 65 requires businesses to provide warnings about significant exposures to chemicals that cause cancer, birth defects or other reproductive harm. CA and MD have led the charge with transparency laws for heavy metals in baby food (CA AB899 & MD SB723).
The two primary sources of contaminants in protein powders are the contaminated soils where ingredients are grown, and the packaging used for these products. At the agricultural level, companies can hold suppliers accountable to minimize pesticide and soil contamination during the growth cycle of their ingredients. However, the good news is that packaging has seen significant improvements in terms of BPA content. Clean Label Project’s testing indicates that BPA has been nearly eliminated from packaging, reflecting the industry’s response to consumer demand and controversy surrounding this chemical.
Clean Label Project contracted an independent analytical chemistry laboratory, Ellipse Analytics, to test 6 industrial chemical panels, including heavy metals and BPA.The heavy metals, arsenic, cadmium, lead, and mercury, were tested by Inductively Coupled Plasma–Mass Spectroscopy (ICP-MS). Bisphenols and pesticides are tested by liquid Chromatography–Tandem Mass Spectroscopy (LC-MS/MS).
Our mission is to empower consumers to see beyond flashy marketing. When it comes to selecting low-contaminant options, our findings reveal that plant-based protein powders generally had the highest levels of detected contaminants, while whey-based protein powders consistently demonstrated lower levels. The data from this protein study, enables consumers to make informed choices that prioritize safety and quality in their dietary supplements. Based on our study, the products with the least lead are whey or collagen-based protein powders that are not chocolate-flavored.
The following companies have protein powders that are Clean Label Project Certified:
Stay tuned as more brands are currently going through the Certification Process.
“The food industry owes their customers an open, honest, and transparent view of how clean their ingredients are,” explains Jaclyn Bowen from Clean Label Project. “Consumers are purchasing supplement and protein products for health and performance, they expect the products to be clean.”
Transparency laws like CA AB899 and Maryland “Rudy Law” is the future for consumer trust and industry change. Clean Label Project has a Transparency certification that displays the results of all certified brands lots providing consumers with the confidence to make the right informed decisions. We believe there is a growing concern about food and supplement safety and a growing demand for transparency.
Currently only one protein company, Puori, is certified to Clean Label Project Transparency Certification but we encourage more brands to seek Transparency Certification providing consumers with the trust they deserve.