What’s in Your CBD Products? New Clean Label Project Peer-reviewed Study Uncovers Some Disturbing Answers
CBD is quickly becoming a consumer phenomenon. Since the 2018 Farm Bill opened the door to hemp-derived cannabidiol (CBD), consumer demand for over-the-counter CBD products, from topicals to edibles, has grown exponentially.
CBD sales in the U.S. alone hit $4.6 billion in 2020, and forecasts project a U.S. market of more than $20 billion by 2025.
A significant portion of the market growth is among consumers seeking medical alternative treatments. According to a 2022 survey, 62 percent of U.S. adults using CBD stated they took it for anxiety and stress. A further 60 percent indicated they used it for pain relief and 48 percent at a doctor’s suggestion.

Yet, despite this rapidly growing market and the use of CBD products by many new consumers for medical purposes who are potentially medically vulnerable, the industry remains significantly underregulated. While the CBD industry has called for strict regulation, the FDA has been slow to act.
So what does the lack of FDA regulations mean for consumers?
Clean Label Project sought to answer that question by conducting a broad study of commercially available CBD products. As is our protocol, we replicated the consumer experience by buying products directly through online and bricks and mortar stores. The products were then sent to the third-party analytical chemistry laboratory, Ellipse Analytics, where they were carefully prepared and tested for the presence of heavy metals, phthalates, and potency levels.
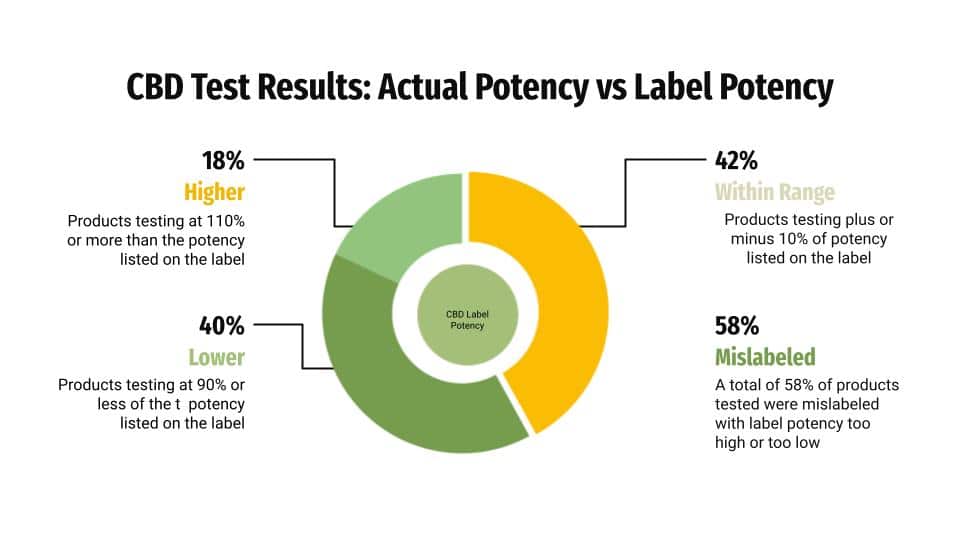
The results published in the Science of the Total Environment journal paint a picture of inconsistency and mislabeling which should raise concerns among consumers about just what they are getting when they use some CBD products.
Nearly 60% of the 516 CBD products tested were mislabeled, with significant discrepancies between the label and actual potency. Tests found that 40 % contained less than 90 % of the CBD indicated on the product label, while 18 % had more than 110 %, and only 42 % of products fell within ±10 % of the CBD claimed on the manufacturer label.
In the 121 edible CBD products tested, trace amounts of heavy metals were detected; lead in 42 %, mercury in 37 %, arsenic in 28 %, and cadmium in 8 %. Four of the edible CBD products exceeded the California Proposition 65 threshold for daily lead consumption of 0.5μg in two servings. Heavy metals are naturally occurring elements with multiple industrial, domestic, agricultural, medical, and technological applications. In larger amounts, they can become toxic or dangerous. Heavy metal toxicity can lower energy levels and damage the functioning of the brain, lungs, kidneys, liver, blood composition, and other important organs.

Phthalates are widely used chemicals in plastics; they are known hormone disruptors and have been linked to endocrine disruption; research over the past decade raises concerns over their impact on human health. The percentage of products with detectable amounts across the four phthalates ranged between 13 % and 80 %.
It is likely that this information is not surprising to the FDA. FDA has seen similar issues with mislabeling and contamination as outlined in its summer 2020 report to congress on CBD products.
However, despite FDA’s own findings and broad industry calls for regulatory clarity, the FDA has yet to promulgate clear standards as they seek to acquire more data.
The Clean Label Project study, which analyzed twice the number of products as the FDA, should serve as a wake-up call for FDA to no longer delay and take steps immediately to protect consumers.
